

Pharmaceutical company Inovio has received a $71 million grant from the Defense Department to scale up an emerging injection technology that will be used to administer its experimental DNA vaccine for COVID-19.
The Pentagon awarded the grant to Inovio Pharmaceuticals in July 2020 to aid in the development of its CELLECTRA 3PSP delivery device, an injection gun that creates an electrical pulse essential to the delivery of its DNA COVID-19 vaccine, called INO-4800.
Unlike the mRNA injection, Inovio’s DNA shot requires a device that administers an electric charge to the injection site to enable its proprietary DNA vaccine plasmids to more efficiently invade the cells, claiming the procedure yields no “observed” side effects or toxicity.
“Hopes remain high for another kind of nucleic-acid vaccine, one that makes use of DNA rather than mRNA. DNA-based vaccines have most of the advantages of mRNA vaccines, yet they produce no significant side effects—and, crucially, they don’t need to be refrigerated. These attributes could make these vaccines a boon to rural and low-resource regions,” IEEE Spectrum magazine reported in May.
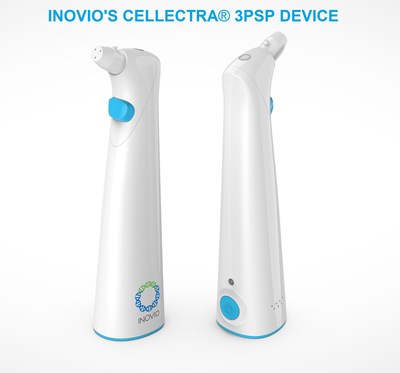
“The company, Inovio Pharmaceuticals, is using a technique known as electroporation, in which an electrical pulse applied to the skin briefly opens channels in cells to allow the vaccine to enter.”
“After a standard vaccine injection, Inovio’s electroporation device, which looks like an electric toothbrush, is held against the skin. At the press of a button, a weak electric field pulses into the arm, opening channels into the cells. The tool gives DNA vaccines the boost they need to work in humans—or so the company says. It’s an engineering solution to a biological problem.”
“If we really have to vaccinate 7 billion people, we might just need every possible technology,” said Margaret Liu, chairman of the board of the International Society for Vaccines.
Inovio CEO Dr. Joseph Kim praised the DoD for their research grant funding in a July 2020 statement and claimed his company hopes to deploy new DNA injection technology by 2021 for use in the worldwide COVID-19 vaccine campaign.
“We look forward to working closely with DoD, JPEO-CBRND and JPL-CBRND-EB to provide much needed protection to DoD personnel and their families through development of a safe and effective vaccine against COVID-19,” Kim said.
“This next generation smart device leverages the efficacy delivery and safety track record of an earlier version that has received CE mark certification and has been used in clinical trials to safely dose more than 2,000 patients in over 7,000 administrations of INOVIO’s DNA medicines.”
“The current DoD contract further supports INOVIO’s large-scale production of devices and arrays to deliver potentially hundreds of millions of doses of INO-4800 next year to combat the global COVID-19 pandemic,” he added.
Notably, Inovio also received a $5 million donation in May 2020 from the Bill & Melinda Gates Foundation to test and scale up the electrical DNA injection device.
How long it would take the Food and Drug Administration to approve such a device and vaccine in time remains to be seen, but given how the FDA handled the rollout of the experimental mRNA injection, it could likely approve Inovio’s injection for Emergency Use for some time before granting it full authorization.

 How Crypto Works?
How Crypto Works?  Why Is Crypto Down? The Truth.
Why Is Crypto Down? The Truth.  Cleveland Clinic Bans Severely Ill Ohio Man From Kidney Transplant Because The Donor Isn’t Vaccinated
Cleveland Clinic Bans Severely Ill Ohio Man From Kidney Transplant Because The Donor Isn’t Vaccinated  LOUISIANA WILL EXPUNGE YOUR CRIMINAL RECORD IF YOU AGREE TO GET VACCINATED
LOUISIANA WILL EXPUNGE YOUR CRIMINAL RECORD IF YOU AGREE TO GET VACCINATED  Kraft Heinz CEO says people must get used to higher food prices
Kraft Heinz CEO says people must get used to higher food prices 


